The first digital pill: innovation or invasion?
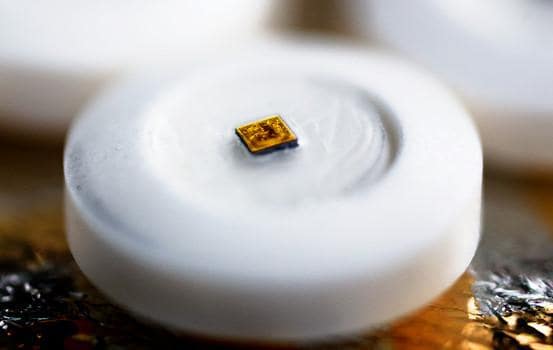
The Food and Drug Administration (FDA) recently approved the first digital pill that tracks if patients have taken their medication. Our experts weighed in on the potential benefits of the new technology, as well as the potential for abuse.

The pill, a version of the antipsychotic Abilify used to treat schizophrenia and other mental illnesses, has an ingestible sensor that communicates with a wearable patch to record date and time of ingestion, as well as other physiological data. Patients can track their activity in a mobile application and permit their doctors and/or caregivers to view the information online.
Many hope the new technology will help to address the longstanding problem of medication nonadherence, which accounts for more than $100 billion in avoidable health care costs each year in the US. “Medication nonadherence is a huge public health challenge in the United States,” said Kevin Volpp, director of the Center for Health Incentives & Behavioral Economics (CHIBE), in an interview with The Philadelphia Inquirer. The new technology adds to a growing list of digital devices used to monitor medicine-taking, such as digital pill bottles. Previous research by Dr. Volpp has shown that automated reminders and physician notification via digital pill bottles are a promising means to help high-risk patients follow their prescribed medication regimen.
However, the digital pill goes a bit further, explained Holly Fernandez Lynch, assistant professor of medical ethics and health policy, in an interview with Wharton Business Radio. “What’s new about [the digital pill] is this is something you swallow, and it’s less easy to game the system.” An electronic pill bottle records that the bottle is opened, but not whether the medication has been taken. This technology is the next step to avoid that kind of gaming.
In the same interview, Robert Field, professor of law and professor of health management and policy at Drexel University, highlighted the pill’s potential to address other public health issues in which medication adherence plays a large part, including antibiotic resistance, infectious diseases (specifically, tuberculosis), and AIDS (antiretroviral drugs). In addition, Fernandez Lynch and Field both agreed that clinical trials are among the most exciting new uses for the digital pill; it could help researchers determine whether a new medication is not effective, or if subjects simply aren’t taking it.
Despite these possible benefits, our experts also expressed concerns about the new technology. One point of debate is whether Abilify is the right product to start with, explained Fernandez Lynch. On one hand, missing a dose of medication for patients with schizophrenia has serious clinical implications, and reminders could be critical. On the other hand, there are also concerns about whether that population has the capacity to consent to this type of medication. Although wearing the patch is voluntary, this may be especially problematic if insurers begin to tie copays and/or benefits to use of the digital pill. For example, if an insurer refuses to cover the drug if a beneficiary doesn’t wear the patch, Fernandez Lynch said, “then we have to start asking: ‘Is it still voluntary? When does it no longer become voluntary?”
Privacy is also a concern, explained Emily Largent, assistant professor of medical ethics and health policy, in a radio interview with The Takeaway. “Because mental health is stigmatized, we’re worried about this particular group of patients being the first group going out,” she said. However, Dr. Largent noted that the new technology has protections built into it to avoid an invasion of privacy. “The monitoring is not continuous, as the sensor is digested and excreted. The patch can be removed and needs to be replaced every seven days anyhow. And patients can instantly remove physicians and family members from seeing their data if they don’t want them to. Patients still have a fair amount of control,” she explained.
The FDA expects an uptick in proposals for new digital pills that treat other conditions. Ultimately, our experts agree that this is an exciting new advance in medication-monitoring technologies. However, it will only work if clear protections are put in place to prevent abuse and the data are used in a way to collaboratively problem-solve with patients for their benefit rather than punishing them for their decisions.
This post originally appeared on Health Policy$ense.